Provide domestic leading comprehensive innovative solutions
Scroll down
We have developed a complete set of proprietary technology platforms covering all aspects necessary for the development and production of transcatheter valve systems. Those R&D technology platforms are interdependent on, complementary to, and synergistic with, each other. We have applied our technological expertise to our BE and SE prosthetic valve stents, repair clips, and other product candidates. We have established a solid underlying technology platform, which makes it possible to continuously develop innovative medical device products with the latest international technology.
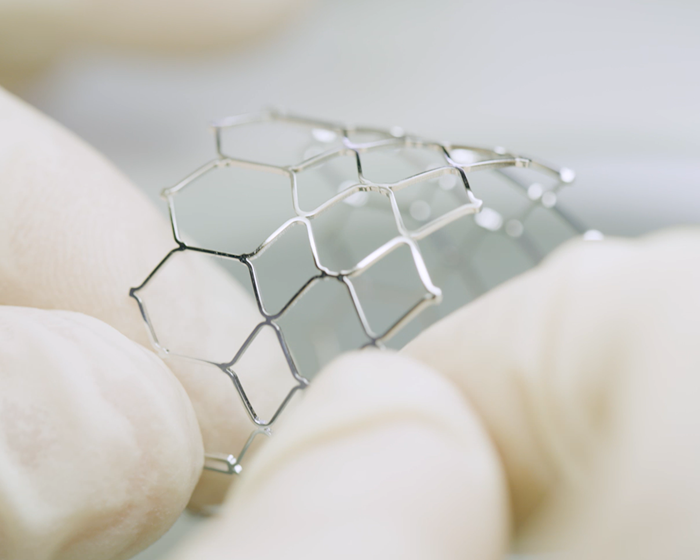
We have mastered the material processing techniques for both cobalt-chromium alloy used in BE products and nitinol alloy used in SE products. We have acquired key pieces of technology crucial to the research, development, and design of all types of stents, as well as their complete processing techniques and production processes, including laser cutting, heat treatment, sandblasting, and polishing. Our inspection and testing capabilities, as well as our R&D, production, and testing equipment, are in compliance with industry and national standards.
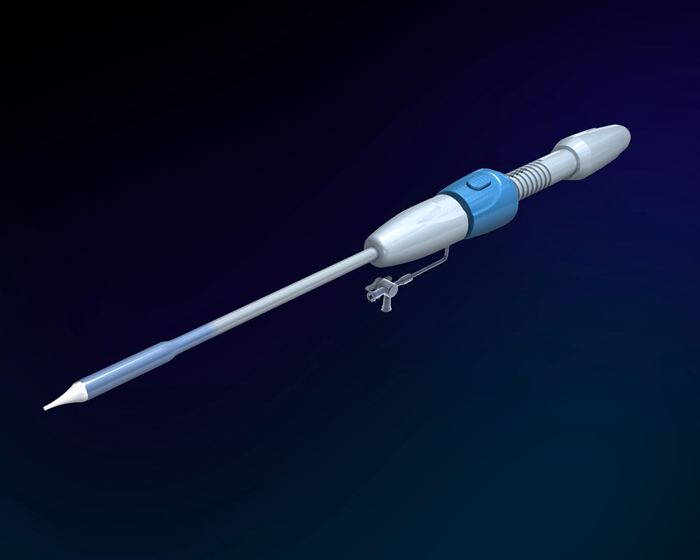
Medical polymer materials are used in the delivery systems of transcatheter valve devices. We have mastered key technologies related to polymer catheter design, structural design of the steerable handle, and the complete production process. We have developed an evaluation and testing protocol for delivery catheters.
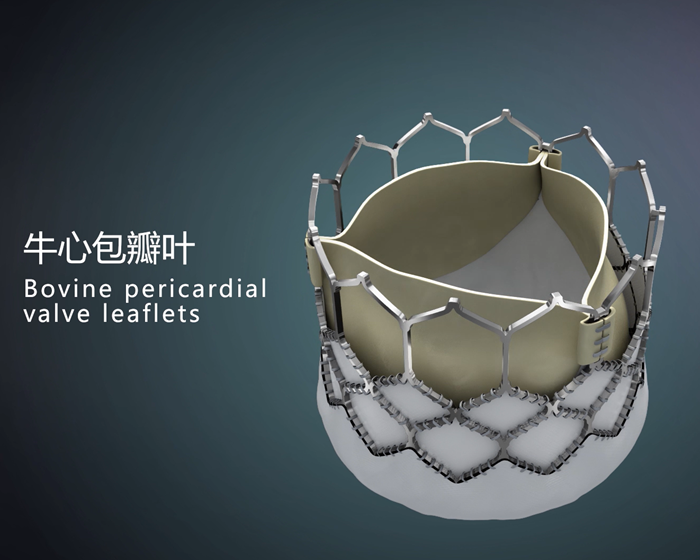
Bovine pericardium is a key raw material for our prosthetic replacement valve product candidates. Its durability is determined by key factors such as the anti-calcification treatment and immunogenicity-removing treatment. To date, bioprosthetic heart valve-manufacturing companies in and out of China are still devoting great amounts of effort to overcoming the major technological challenges associated with those treatments. Our R&D and manufacture capabilities, which are in compliance with industry standards, cover every step of prosthetic valve manufacturing, including raw bovine pericardium sourcing and processing, leaflet design, tailoring of processed bovine pericardium, and the eventual suturing of tailored bovine pericardium. Specifically, our tissue engineering technique produces excellent anti-calcification and immunogen removal performances, and it is central to our prosthetic valve technology.
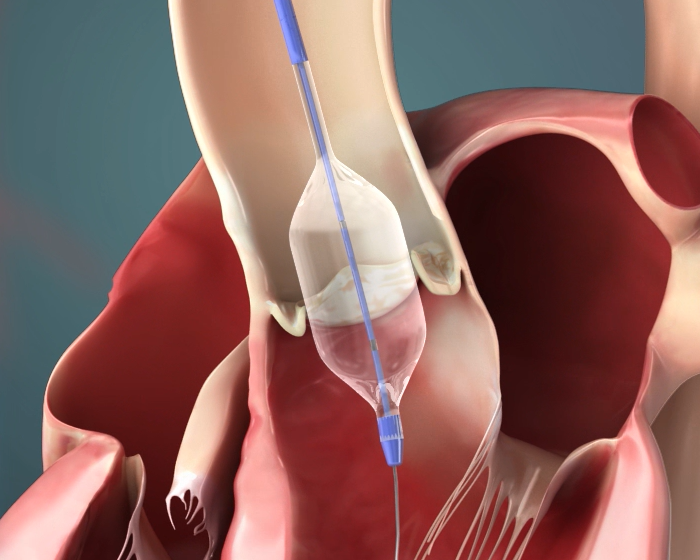
Large balloon is a core piece of technology necessary for our BE product candidates. It requires the full set of R&D and production techniques of balloon structural design, balloon stretching, forming, folding, and shaping. We have acquired the R&D, manufacture, and testing capabilities for balloons of all sizes.

Address: Building 6, Lane 500, Furonghua Road, Zhoupu Town, Pudong New Area, Shanghai
Tel: +86 21 20788668
Fax: +86 21 20788659

Copyright©Shanghai NewMed Medical Co., Ltd 沪ICP备16013125号-2  沪公网安备31011502016148
沪公网安备31011502016148

